The placenta isn’t a wall. It’s not a filter that blocks everything bad. It’s more like a busy, living checkpoint - letting some things through, blocking others, and changing how it works as your pregnancy progresses. When you take a pill during pregnancy, it doesn’t just disappear. It enters your bloodstream, travels to the placenta, and from there, it can reach your baby. Some drugs cross easily. Others barely make it. And the effects on the fetus? They can be minor, serious, or even life-changing.
What Makes a Drug Cross the Placenta?
Not all medications behave the same way. The key factors are simple but powerful: size, solubility, and how the body handles them. Drugs with a molecular weight under 500 daltons slip through the placental barrier more easily. That’s why small molecules like alcohol (46 Da) and nicotine (162 Da) reach the fetus almost as quickly as they reach your blood. Within an hour, fetal levels can match maternal levels. Lipid solubility matters too. If a drug dissolves well in fat, it slips through cell membranes like butter on toast. Drugs with a log P value above 2 are far more likely to cross. On the flip side, large or water-soluble drugs - like insulin (5,808 Da) - barely get through. Less than 0.1% of maternal insulin reaches the fetus. Ionization plays a big role. At the body’s normal pH of 7.4, drugs that are charged (ionized) struggle to cross. For example, a drug that’s mostly ionized at this pH might have its placental transfer cut by 80-90%. That’s why some antibiotics and painkillers have limited fetal exposure - not because they’re safe, but because they can’t get there easily. Protein binding is another hidden factor. Only the unbound, free fraction of a drug can cross. Warfarin, for instance, is 99% bound to proteins in your blood. So even though it’s small and fat-soluble, very little actually reaches the baby.The Placenta Isn’t Just a Passive Gate - It’s an Active Barrier
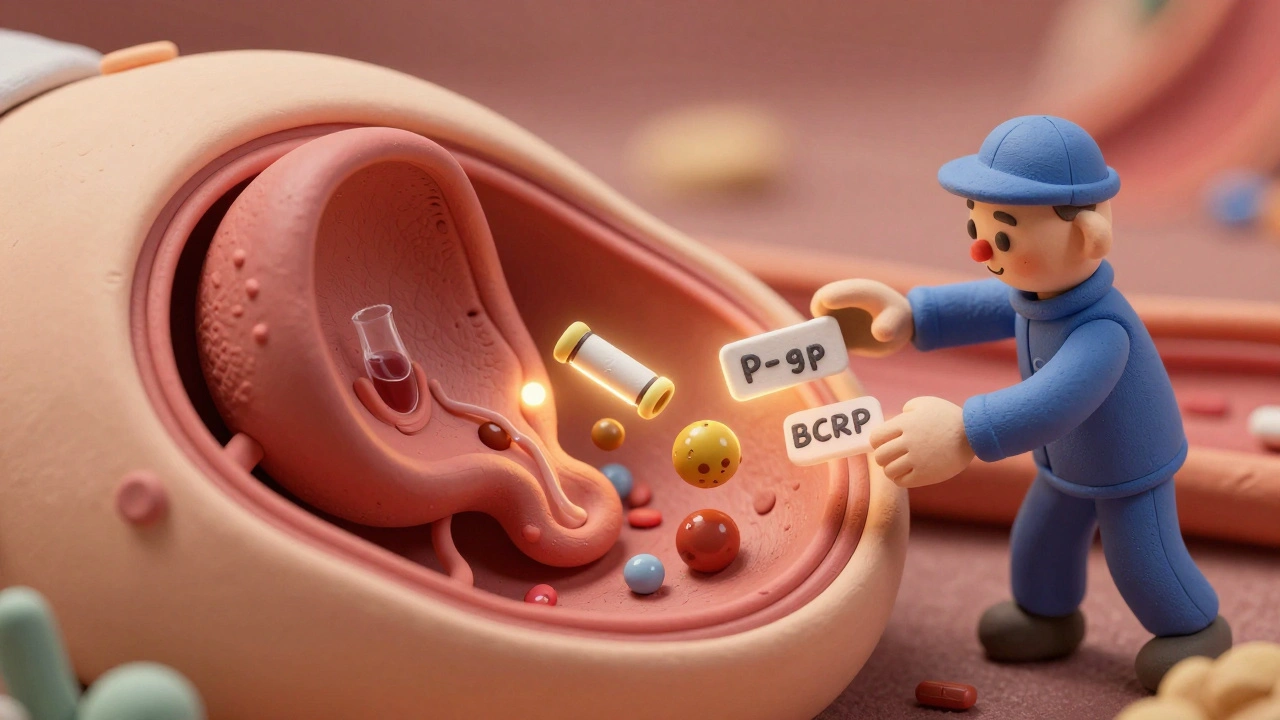
For years, scientists thought the placenta was mostly a passive filter. Now we know it’s packed with pumps and guards. Two major transporter proteins - P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) - actively push drugs back toward the mother’s side. Think of them as bouncers at a club, kicking out unwanted guests. This is why some HIV drugs, like lopinavir and saquinavir, show such low fetal exposure. In studies using human placenta tissue, blocking P-gp increased fetal levels by up to 2.3 times. Without those pumps, more drug gets through. But with them? The fetus gets only about 60% of the mother’s concentration, even when the mother is on a full dose. Even more surprising? Some drugs actually compete with each other. Methadone and buprenorphine, used to treat opioid use disorder in pregnancy, can block BCRP and P-gp. That means if you’re on one of these and also take a chemotherapy drug like paclitaxel, the chemo might cross more easily than expected - raising the risk of fetal toxicity.How Gestational Age Changes Everything
The placenta isn’t the same in week 8 as it is in week 38. Early in pregnancy, the barrier is thinner, less organized, and has fewer transporter proteins. That means more drugs get through during the first trimester - precisely when the baby’s organs are forming. This is why exposure to thalidomide in the first 6-8 weeks caused severe limb defects, while later exposure didn’t. By the third trimester, the placenta becomes more selective. Efflux transporters like P-gp and BCRP are fully active. Tight junctions between cells tighten up. That’s good for shielding the baby from toxins - but it also means some needed medications might not reach the fetus effectively. Research shows first-trimester placentas are 2-3 times more permeable to small molecules than term placentas. Yet most drug studies are done on placentas from full-term births. That’s a huge gap. We’re using data from late pregnancy to make decisions about early pregnancy - when the risks are highest.
Real-World Examples: What Drugs Actually Reach the Fetus?
Let’s look at real medications and what we know about their fetal exposure:- SSRIs (e.g., sertraline): Cord-to-maternal ratios of 0.8-1.0 mean the baby gets nearly as much as the mother. About 30% of exposed newborns show temporary symptoms like jitteriness, feeding trouble, or mild breathing issues - known as neonatal adaptation syndrome.
- Methadone and buprenorphine: Fetal levels reach 65-75% of maternal levels. This leads to neonatal abstinence syndrome (NAS) in 60-80% of infants, requiring weeks of hospital care.
- Phenobarbital: Crosses easily, with near-equal fetal levels. Used for seizures, it’s effective but linked to developmental delays if used long-term.
- Valproic acid: Crosses readily (cord-to-maternal ratio 0.9-1.0). Associated with a 10-11% risk of major birth defects - including spina bifida and heart problems - compared to 2-3% in the general population.
- Zidovudine (AZT): Unlike most HIV drugs, it uses specific transporters to cross efficiently. Fetal levels reach 95% of maternal levels, which is why it’s used to prevent mother-to-child HIV transmission.
- Digoxin: Despite being a heart drug, it crosses poorly - and even when you give inhibitors like verapamil, transfer doesn’t increase. This shows transporter specificity: not all blockers work on all drugs.
Why Animal Studies Don’t Always Tell the Whole Story
Many drug safety studies are done in mice or rats. But their placentas are structurally different. In some cases, rodent placentas are 3-4 times more permeable than human ones. A drug that looks safe in mice might be dangerous in humans - or vice versa. That’s why researchers now rely on dually perfused human placenta models - where a donated placenta is kept alive and tested with real drugs. These models show what actually happens in human tissue. Even better are placenta-on-a-chip systems: tiny devices that mimic the placental barrier with human cells. One study showed glyburide (a diabetes drug) transfer in these chips matched real placenta data within 1%. Still, these systems can’t replicate the full complexity of a living pregnancy - immune signals, hormone changes, fetal metabolism. That’s why experts warn: we still don’t fully understand what happens in the first trimester.