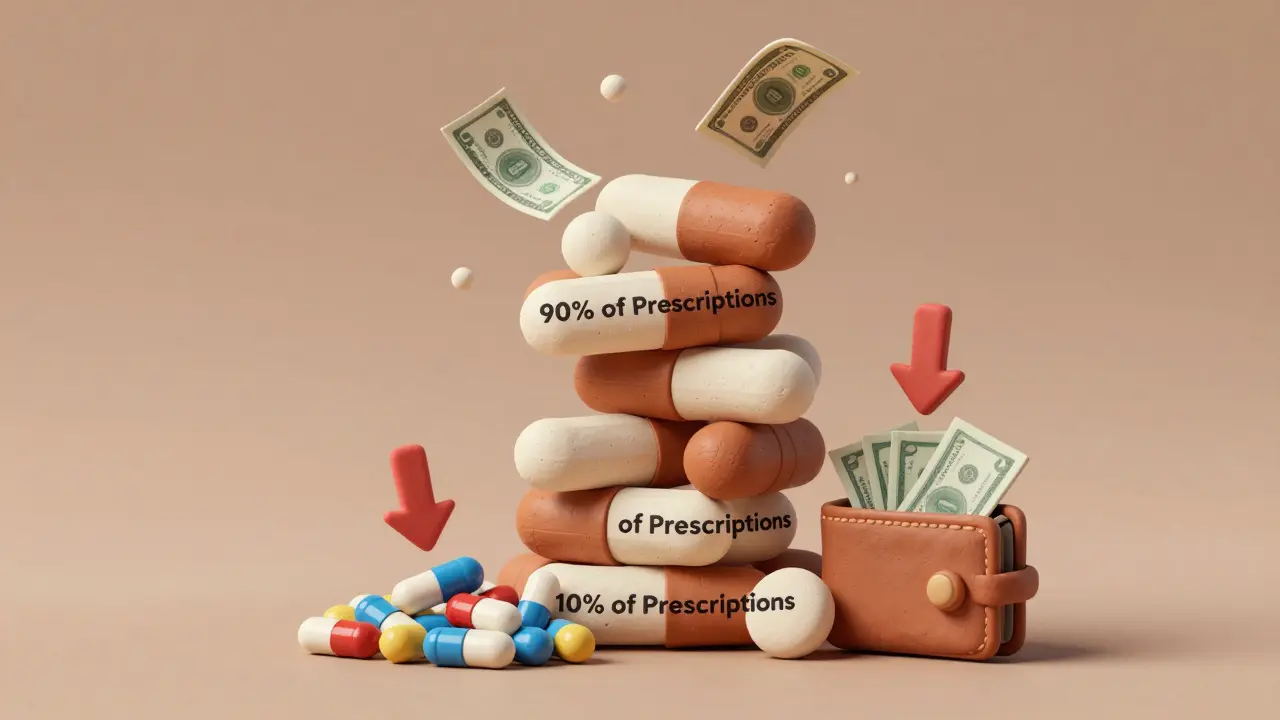
Every year, Americans fill over 4 billion prescriptions. Ninety percent of those are for generic drugs. And yet, these medications account for just 12% of total prescription spending. Meanwhile, brand-name drugs, which make up only 10% of prescriptions, consume 88% of the money spent on prescriptions. This isn’t a mistake. It’s the clearest sign that generics are the most powerful tool we have to control rising drug costs.
What’s Really Saving Money?
In 2024, generic medications saved the U.S. healthcare system $482 billion. That’s not a guess. It’s from the Association for Accessible Medicines (AAM) a nonprofit that tracks the economic impact of generic and biosimilar drugs and IQVIA Institute a leading healthcare data and analytics firm. The numbers don’t lie: generics filled 3.9 billion prescriptions but cost only $98 billion. Brand-name drugs? Just 435 million prescriptions, but $700 billion in costs.
That gap-$482 billion-is what happens when competition enters the market. Once a patent expires, multiple manufacturers can make the same drug. Prices drop fast. A drug that once cost $100 a month might fall to $5, then $2. Patients pay less. Insurance companies pay less. Medicare and Medicaid pay less. Everyone wins-except the companies that used to hold exclusive rights.
Biosimilars: The Next Big Wave
Generic drugs aren’t just for pills. They’re now here for biologics-complex, injectable drugs made from living cells. These are the expensive ones: Humira, Enbrel, Stelara. When their patents expire, the first wave of biosimilars drugs that are highly similar to biologic products but not exact copies hit the market. And they’re cutting prices by more than 80%.
In 2024, Humira biosimilars went from being used in just 3% of cases to 28%. That jump came after health plans started pushing them hard-offering better coverage, lowering copays, and making switching easier. The result? Billions saved in a single year. Stelara, a $6 billion drug, now has seven biosimilars approved. Once they’re fully adopted in 2026, they’re projected to save $4.8 billion annually.
But here’s the problem: 90% of biologics that will lose patent protection over the next ten years have zero biosimilars in development. That’s a $234 billion missed opportunity. The infrastructure is there. The science is proven. The demand is real. What’s missing is the urgency from manufacturers and payers to get started.
Why Brand Drugs Cost So Much
Why do brand-name drugs cost three times more in the U.S. than in other wealthy countries? It’s not because they’re better. It’s because the system lets them. Brand manufacturers use tactics like "pay for delay" agreements where brand companies pay generic makers to delay entering the market-a practice that cost the system over $1.2 billion in settlements last year alone.
One study found that in 2024, a single drug had 17 different patent extensions, each delaying a generic version. These aren’t accidents. They’re legal maneuvers. The Food and Drug Administration (FDA) the federal agency responsible for approving drugs in the U.S. clears generics quickly-but the courts and contracts slow them down. The result? Patients wait years to get affordable versions of life-saving drugs.
Compare that to insulin. When public pressure mounted, Eli Lilly dropped the price from $275 to $25 per vial. That wasn’t because the cost of making it changed. It was because the public called them out. The same pressure can-and should-apply to other drugs.

How Much Are Patients Really Saving?
For many, generics aren’t just a cost-saver. They’re the difference between taking medicine and skipping it. According to GoodRx a prescription price comparison service, 1 in 12 Americans have medical debt from prescription costs. And in nearly every case, the debt came from brand-name drugs.
One Reddit user wrote: "Switching to generic albuterol saved me $300 a month on my asthma inhaler." Another said, "My dad’s blood pressure med went from $140 to $8. He started taking it again." These aren’t anecdotes. They’re the reality for millions.
Medicare data shows that less than 1% of beneficiaries who hit the catastrophic coverage phase use only generics. That means nearly everyone hitting that threshold is paying for expensive brand drugs. If they had switched earlier, they’d have avoided the spike in out-of-pocket costs entirely.
The Bigger Picture: Where Savings Are Going
The U.S. spends $776 billion on prescription drugs in 2025-and that number is climbing. Without generics, it would be over $1.2 trillion. Generic drugs are the only part of the healthcare system that consistently reduces spending year after year. While hospital costs, doctor visits, and insurance premiums keep rising, generic drug spending has actually gone down since 2019-even as more generics were launched.
That’s because competition works. When multiple companies make the same drug, they fight for market share by lowering prices. It’s basic economics. And it’s why countries with faster generic approval systems spend far less on drugs.
Health plans are catching on. Private-label biosimilars-where insurers or pharmacy benefit managers create their own versions-are growing fast. They’re not just cheaper. They’re more predictable. One health plan cut its Humira costs by 60% in one year by switching entirely to biosimilars.

What’s Holding Back More Savings?
It’s not science. It’s policy. And it’s inertia.
The Inflation Reduction Act a U.S. law that allows Medicare to negotiate drug prices is already saving money by capping insulin at $35 for Medicare users. By 2027, that cap will extend to commercial plans. That’s huge. But it’s just one drug. The law allows Medicare to negotiate 30 drugs per year starting in 2026. That could save $500 billion over ten years.
But here’s the catch: if those negotiated prices were extended to Medicaid and private insurers, total savings could hit $1 trillion. Right now, only Medicare is forced to pay less. Everyone else? Still paying full price.
And then there’s the biosimilar void. Why aren’t companies rushing to make biosimilars for the next 100+ drugs losing patents? Because the process is expensive. Because the regulatory path is unclear. Because brand companies still have legal tricks to delay entry. Without stronger incentives, we’ll keep missing out.
What Needs to Change
Three things will unlock the next wave of savings:
- Fast-track biosimilar approvals-the FDA has the power to speed this up. It needs to.
- Ban "pay for delay" deals-these are anti-competitive. Congress has tried to stop them before. It needs to succeed.
- Expand price negotiation-if Medicare can negotiate, why not Medicaid? Why not private insurers? The savings would be massive.
Health plans need to stop treating biosimilars as "second choice." They should make them the default. Pharmacists need better tools to switch patients without confusion. Patients need clear information: "This generic works the same. It’s cheaper. Let’s switch."
The system isn’t broken. It’s being manipulated. And the people paying the price aren’t the CEOs or the investors. They’re the ones with prescriptions in hand, wondering if they can afford to fill them this month.
How much do generic drugs actually save the U.S. healthcare system?
In 2024, generic medications saved the U.S. healthcare system $482 billion. That’s up from $445 billion in 2023. These savings come from the fact that generics make up 90% of prescriptions but only 12% of total drug spending. Brand-name drugs, which account for just 10% of prescriptions, make up 88% of costs.
Are biosimilars as effective as brand-name biologics?
Yes. Biosimilars are not exact copies, but they are scientifically proven to have no meaningful difference in safety, purity, or potency compared to the original biologic. The FDA requires rigorous testing before approval. Thousands of patients have switched to biosimilars with no loss in effectiveness. In fact, studies show they work just as well in treating conditions like rheumatoid arthritis, Crohn’s disease, and cancer.
Why do some pharmacies refuse to substitute generics?
Sometimes, it’s because the prescriber wrote "do not substitute" on the prescription. Other times, it’s because the pharmacy’s system is outdated or the PBM (pharmacy benefit manager) doesn’t incentivize switching. In rare cases, a brand-name manufacturer pays the pharmacy to keep selling their expensive version. Patients should always ask: "Is there a generic? Can I switch?" Most of the time, the answer is yes-and it’ll save money.
Can I trust generic drugs to work as well as brand names?
Absolutely. The FDA requires generic drugs to have the same active ingredients, strength, dosage form, and route of administration as the brand. They must also meet the same strict manufacturing standards. The only differences are in inactive ingredients-like fillers or dyes-which don’t affect how the drug works. Millions of Americans rely on generics every day. They’re not second-rate. They’re the standard.
What’s the "biosimilar void," and why does it matter?
The "biosimilar void" refers to the fact that 90% of brand-name biologics that will lose patent protection in the next 10 years have no biosimilar in development. That means $234 billion in potential savings could vanish because no company is stepping up to make cheaper alternatives. This gap exists because biosimilars are expensive and complex to develop, and brand manufacturers use legal tactics to delay competition. Without policy changes, patients will keep paying high prices long after patents expire.
How does the U.S. compare to other countries in drug pricing?
Americans pay more than three times what other wealthy countries pay for the same brand-name drugs. Countries like Canada, Germany, and the UK negotiate prices directly with manufacturers or set price caps. The U.S. doesn’t. That’s why generics and biosimilars are even more critical here-they’re the only real check on runaway drug costs.
What role do pharmacy benefit managers (PBMs) play in generic savings?
PBMs are middlemen between insurers, pharmacies, and drug makers. They often push for generics because they’re cheaper. But they also receive rebates from brand-name manufacturers, which can create conflicts of interest. Some PBMs still favor expensive drugs if the rebate is high enough. Transparency and reform are needed to make sure PBMs prioritize patient savings over corporate profits.
What Comes Next?
The next decade will decide whether the U.S. keeps wasting billions on overpriced drugs-or finally uses the tools we already have. Generics and biosimilars are proven. They’re safe. They’re available. What’s missing is the political will to remove the barriers that keep them from reaching every patient.
Every time someone switches to a generic, a family saves hundreds. Every time a biosimilar enters the market, a health plan avoids a spike in costs. Every time a patent expires without a competitor, a billion dollars slips away.
The solution isn’t complicated. It’s simple: let competition work. Stop delaying generics. End pay-for-delay deals. Expand price negotiation. And make biosimilars the default, not the afterthought.
The money is there. The science is solid. The patients are waiting.