SSLR Risk Assessment Tool
Serum Sickness-Like Reaction Assessment
This tool helps clinicians assess the probability of Serum Sickness-Like Reaction (SSLR) based on key clinical features. SSLR typically appears 1-21 days after antibiotic exposure, most commonly with cefaclor in children under 10 years old.
Risk Assessment Results
Next Steps
Imagine giving a child a short course of antibiotics for an ear infection, only to see a rash and swollen joints appear a week later. It feels like an allergic shock, but the usual allergy tests come back clean. That scenario is classic for Serum Sickness-Like Reaction, a delayed hypersensitivity that mimics true serum sickness yet follows a very different biological path.
What Exactly Is a Serum Sickness‑Like Reaction?
Serum Sickness‑Like Reaction (SSLR) is a distinct, immune‑mediated drug reaction that typically shows up 1-21 days after exposure to certain antibiotics, most often cefaclor. Unlike classic serum sickness, which relies on circulating immune complexes, SSLR does not produce detectable complexes or complement consumption. The condition emerged in pediatric literature during the 1980s and was formally separated from true serum sickness in 1987 after researchers at the University of Minnesota linked it specifically to antibiotics rather than heterologous serum products.
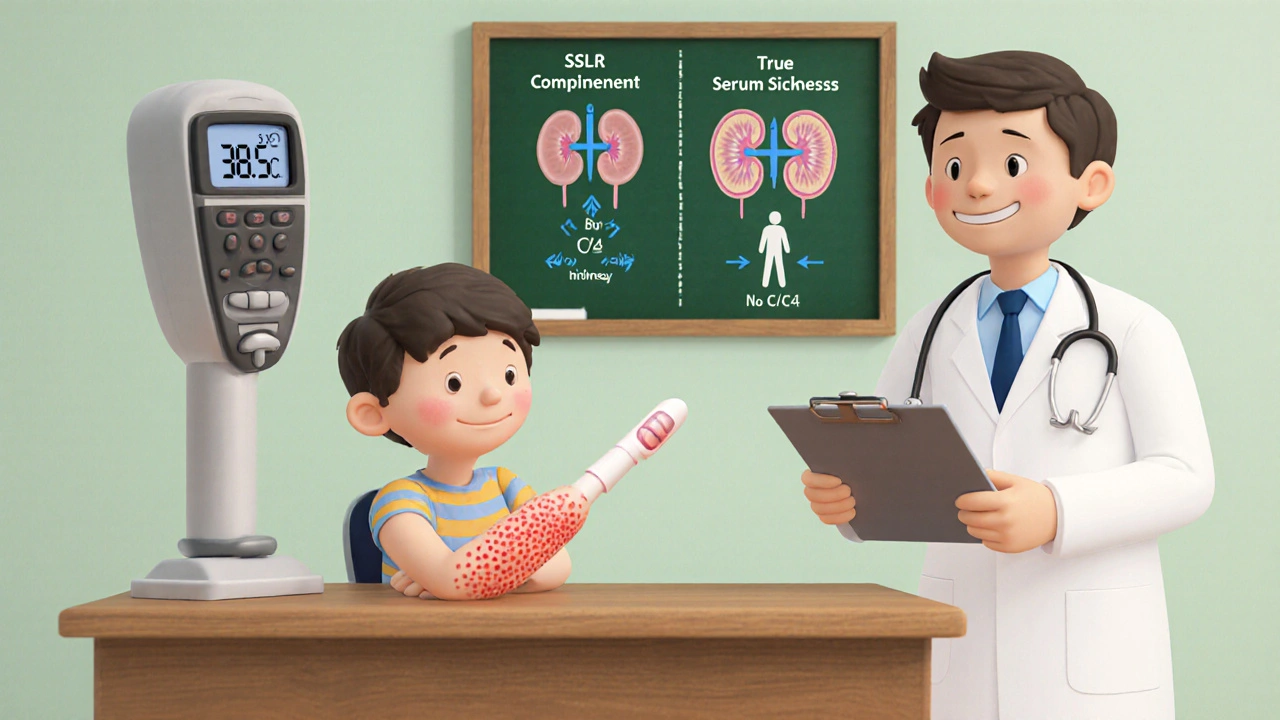
How Does SSLR Differ From True Serum Sickness?
Both conditions share a triad of rash, fever, and joint pain, but the devil is in the details. True serum sickness is a Type III hypersensitivity with immune‑complex deposition, low complement (C3/C4), and often kidney involvement. SSLR, on the other hand, presents with normal complement levels, no detectable immune complexes, and virtually no renal signs.
| Feature | Serum Sickness‑Like Reaction | True Serum Sickness |
|---|---|---|
| Trigger | Cefaclor, amoxicillin, other β‑lactams | Antiserum, antivenom, monoclonal antibodies |
| Onset | 7-10 days (range 1-21) | 5-14 days |
| Immune complexes | Absent | Present |
| Complement C3/C4 | Normal | Low |
| Renal involvement | None | 15‑25% develop proteinuria |
| Resolution | 3‑7 days after drug stop | ~14 days, may need steroids |
| Age distribution | 78% under age 10 | Adults predominate |
The table above makes it clear why conflating the two can lead to unnecessary lifelong antibiotic avoidance.
Who Is Most Likely to Develop SSLR?
Data from Cincinnati Children’s Hospital (2022) show that about 0.2‑0.4 cases arise per 1,000 antibiotic prescriptions. Children ages 6 months to 6 years bear the brunt, accounting for roughly three‑quarters of reported events. Cefaclor alone is responsible for 65‑80% of pediatric SSLR cases, while amoxicillin and other β‑lactams account for the remainder.
Genetic clues are surfacing too. A 2023 pharmacogenomics study linked the CYP2C9*3 polymorphism to roughly 72% of SSLR cases, suggesting that a child’s drug‑metabolizing profile can tip the balance toward a delayed reaction.
What Does SSLR Look Like Clinically?
The classic triad shows up in most patients:
- Urticarial rash - migratory, intensely itchy, lasting minutes per spot before moving.
- Fever - typically 38‑39 °C, present in about 85% of cases.
- Arthralgia/arthritis - symmetric involvement of knees, wrists, ankles in ≈ 72%.
Additional signs can include lymphadenopathy (≈ 45%), malaise (68%), and myalgia (30%). Notably absent are the renal signs that flag true serum sickness, and there’s no palpable purpura that would hint at vasculitis.
How Do You Diagnose SSLR?
The diagnosis is largely clinical, anchored by timing (1‑21 days after the drug) and the symptom triad. Laboratory workup is useful for ruling out mimics:
- Complete blood count - often normal, may show mild eosinophilia.
- Serum complement C3/C4 - stays within normal range.
- Urinalysis - no proteinuria, differentiating from true serum sickness.
- Cryoglobulins & immune complex assays - negative.
When in doubt, a skin biopsy can be performed; SSLR histology shows perivascular lymphocytic infiltrate without true vasculitis, whereas serum sickness would reveal immune complex deposition.
Management: What Should You Do When SSLR Is Suspected?
The first step is simple but critical: stop the offending antibiotic within 24 hours of symptom recognition. Early withdrawal shortens the illness dramatically.
Symptom control follows standard guidelines:
- Second‑generation antihistamines (e.g., cetirizine 0.25 mg/kg every 12 h) for pruritus.
- NSAIDs such as ibuprofen 10 mg/kg every 8 h for arthralgia.
- Oral prednisone 1 mg/kg/day tapered over 7‑10 days is reserved for severe, function‑limiting cases (about 5% of patients).
Most children improve within three days of drug cessation; 92% resolve completely by day 7. A small minority (≈ 8%) may experience intermittent rash or joint soreness for up to three months.
Future Antibiotic Use: Will the Child Need to Avoid All β‑Lactams?
One of the biggest misconceptions is that SSLR equals a lifelong penicillin allergy. In reality, the allergic reaction is drug‑specific. AAAAI 2022 guidelines state that cross‑reactivity to other β‑lactams is low-about 89% of patients tolerate alternative cephalosporins after an SSLR episode.
Allergist‑directed oral challenge protocols, typically performed 6‑36 months after the initial reaction, show a 92% success rate for non‑cefaclor antibiotics. This means a child who reacted to cefaclor can safely receive amoxicillin or cefuroxime later, provided a qualified specialist oversees the rechallenge.

Avoiding Misdiagnosis: Common Pitfalls and How to Overcome Them
Studies reveal that up to 30% of SSLR cases are initially labeled as viral exanthems or simple acute urticaria. Mislabeling leads to broader‑spectrum antibiotic use, higher healthcare costs, and unnecessary allergy cards.
Key red flags that point toward SSLR instead of a viral rash are:
- Onset > 48 h after starting the drug.
- Presence of symmetric joint swelling without carditis.
- Normal complement and urinalysis.
Electronic health record alerts using AI have shown 88% sensitivity in flagging potential SSLR cases, a promising tool for busy clinicians.
Emerging Research: Where Is the Field Heading?
Several avenues are reshaping how we think about SSLR:
- Biomarker discovery - Urinary metabolite panels are nearing 94% sensitivity for distinguishing SSLR from true allergy (UC San Diego trial, 2024).
- Genetic screening - Ongoing PREDICT study (2023‑2026) aims to embed CYP2C9 genotyping into pediatric prescribing workflows.
- Regulatory updates - FDA draft guidance (2023) now requires drug labels to explicitly mention SSLR as a separate adverse reaction.
- ICD‑11 coding - The 2024 International Consensus Document assigned code RA43.1, improving epidemiologic tracking.
These advances promise faster diagnosis, targeted avoidance, and fewer blanket antibiotic bans.
Quick Checklist for Clinicians
- Identify drug exposure timeline (1‑21 days).
- Confirm triad: migratory urticaria, fever, symmetric arthralgia.
- Order complement, urinalysis, and immune‑complex panel to rule out true serum sickness.
- Discontinue suspected antibiotic immediately.
- Provide antihistamine + NSAID; consider steroids for severe cases.
- Document as “Serum Sickness‑Like Reaction” in EHR, not “penicillin allergy.”
- Plan oral challenge for alternative β‑lactams after 6 months.
Frequently Asked Questions
How long after stopping the antibiotic will symptoms last?
Most children feel better within 3 days, and 92% are symptom‑free by day 7. A minority may have intermittent rash for weeks to months.
Can an adult get SSLR?
Yes, adults can develop SSLR, but the incidence is lower-about 22% of reported cases involve patients over 18 years.
Is skin testing useful for SSLR?
Skin testing is generally not helpful because SSLR is not IgE‑mediated. Diagnosis rests on clinical timing and exclusion of other causes.
Should I avoid all cephalosporins after a cefaclor‑triggered SSLR?
No. Studies show that 89% of patients tolerate a different cephalosporin after a cefaclor‑related SSLR, especially when a supervised oral challenge is performed.
What labs are most telling for SSLR?
Normal complement (C3/C4), negative immune‑complex assays, and a clean urinalysis are key. Elevated eosinophils may be present but are not diagnostic.
Understanding SSLR changes how we label drug reactions and, more importantly, how we keep children from being stuck with overly broad allergy restrictions. By recognizing the timing, symptoms, and lab profile, clinicians can stop the offending drug, treat symptoms, and safely reintroduce other β‑lactams when needed.